Novel Methodologies for Modern Drug Development.
by Amy Cooke
INTRODUCTION
Global regulatory authorities are advocating early communication for developers of novel methodologies for use in the nonclinical and clinical stages of drug development. Novel methodologies and biomarkers are becoming increasingly important in improving the success of drug development programmes and thereby accelerating the availability of new therapeutics. This article focuses on the procedure offered by the European Medicines Agency (EMA), but due consideration should be given to similar initiatives in other territories (e.g. CDER Biomarker Qualification Program in the United States, which will be the subject of a follow-up paper).
To support the development of novel methodologies for a specific use in the research and development of drugs, the EMA established a scientific advice procedure for the qualification of these methods in 2008. The EMA’s Committee for Medicinal Products for Human Use (CHMP), on the basis of recommendations by the Scientific Advice Working Party (SAWP), will either offer an opinion on the acceptability of a specific use of a method or advice on protocols and methods intended to develop a novel method. The EMA also encourage that developers seek parallel advice from the EMA and the US Food and Drug Administration (FDA) on novel methodologies in order to obtain concurrence in global development programmes.
The novel methodologies procedure takes approximately 160 or 250 days for qualification advice or a qualification opinion, respectively, however within the procedure, the option for clock stops means the total duration is likely to be longer. The EMA has published several guidance documents to assist developers adopting novel methodologies. These guidance documents summarise the procedure, documentation requirements and essential considerations for a successful qualification outcome. This article will provide an overview of the practical considerations and potential benefits of this procedure.
RELEVANT PARTIES
Within the EU, the EMA’s CHMP is responsible for providing the qualification advice or qualification opinion, based on recommendations from the SAWP. For each qualification procedure the CHMP will appoint a specialised group, named a “qualification team,” which is led by a coordinator who is a CHMP and/or SAWP member.
This qualification team is selected depending on the expertise required for the specific request, including the technology supporting the novel methodology and the context of the proposed use of this method, and is usually made up of one CHMP member, one SAWP member and at least three experts. An EMA Scientific Officer will also be assigned for each procedure, who acts as the Applicant’s contact point and supports successful validation of the request. The scientific community is also involved in later stages of a qualification opinion, with a public consultation held to ensure the views of the scientific community are taken into consideration.
QUALIFICATION ADVICE VS QUALIFICATION OPINION
Depending on the stage of development, the CHMP will provide qualification advice or a qualification opinion on a specific use of the proposed novel methodology. The Applicant can propose which pathway is most appropriate, but the CHMP will make the final decision. For qualification advice, protocols and future development plans are submitted along with any existing data, and the CHMP will provide advice on development of the method towards qualification, based on scientific rationale and preliminary data. Along with the advice, the CHMP can also propose a letter of support for promising novel methods that cannot yet be qualified; letters of support are released as public documents. For a qualification opinion, protocols, study reports and any supportive data are submitted to establish the use of the novel method for a specific purpose. The CHMP will provide an opinion, which states the acceptability of a specific use of the proposed method in research and development, based on the data presented. The qualification opinion is released as a public document. The EMA has published a guidance document summarising some of the essential considerations for successful qualification of novel methodologies (EMA/750178/2017). The benefit of this system is that no ‘negative’ opinion on a novel method will be published – the outcome options are qualification opinion which is the Agency’s endorsement of the novel method, or qualification advice when the Agency cannot yet confirm the acceptability of the novel method, which is provided to the Applicant only.
ELIGIBILITY
To be eligible for the novel methodologies procedure, the Applicant must be developing a novel methodology for a specific use in the research and development of medicinal products. The novel methodology can be developed for either nonclinical or clinical studies. For example, a clinical novel methodology could be a new diagnostic (e.g. a biomarker or an imaging method) for selecting patients for clinical trials, which is particularly important in the field of targeted medicine development, or an outcome measure to act as an endpoint for a clinical trial. An example of a nonclinical novel methodology would be a new animal model for in vivo studies of a drug under development for a specific indication.
DOSSIER STRUCTURE
The EMA has provided detailed guidance on the information that should be included in the novel methodologies procedure request dossier (EMA/CHMP/SAWP/72894/2008). The key information that is required is as follows:
Table of contents
Executive summary
A statement of the need for (and potential impact of) the novel methodology
Methodology and results
Conclusion
References and appendices
The dossier structure is the same for a qualification advice or a qualification opinion procedure. However, for qualification advice, the dossier may include draft protocols and development plans, while for a qualification opinion the dossier is likely to contain more data from completed studies. Along with the dossier, the Applicant submits a completed letter of intent, for which a template is available from the EMA website.
PROCEDURAL ASPECTS, TIMELINES & OUTCOMES
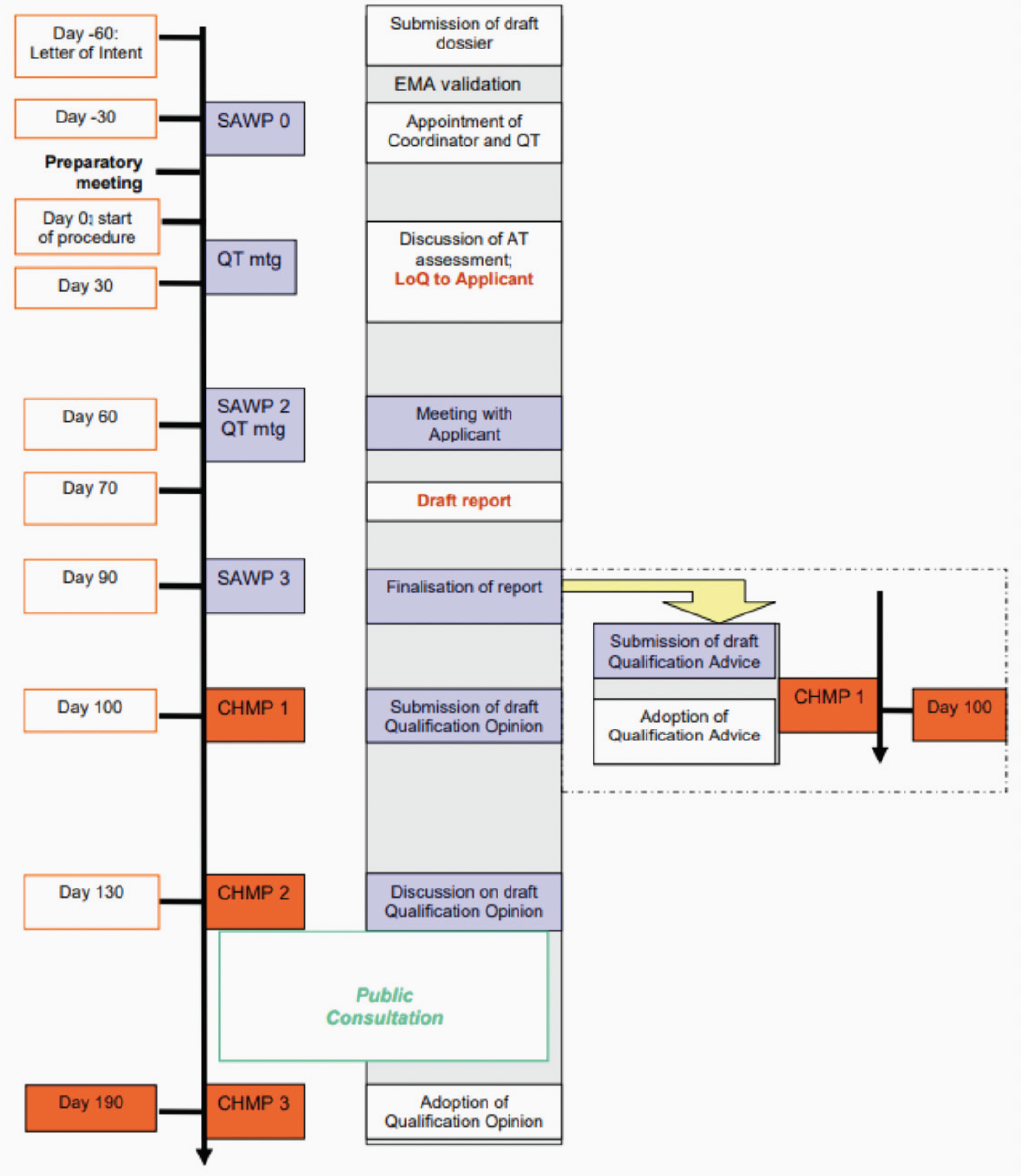
In the same guidance, the EMA has summarised the steps in the procedure and the associated timelines. In brief, the request is submitted according to the published submission timeline, following which there is a 60-day pre-submission phase. In common with other EMA procedures, during a ‘pre-submission phase’, the dossier is validated, the qualification team is appointed and a preparatory meeting is held. Following the successful outcome of pre-submission, the procedure then starts on Day 0 and the EMA appointed coordinators begin their review of the dossier. During the evaluation phase, the qualification team assesses the data and presents their initial assessment at a meeting with SAWP. The Applicant will then be sent a list of questions and invited to attend a discussion meeting. After this meeting. The CHMP will confirm whether qualification advice or a qualification opinion will be given. For qualification advice, the Applicant will receive a written scientific advice letter providing input on the studies required to generate data for a successful qualification opinion in future. As discussed above, a letter of support may be published for promising methods. For a qualification opinion, the draft opinion is published for a 6-week public consultation, following which the final qualification opinion of the acceptability of the novel methodology for a specific use will be published. Please refer to Figure 1 for a flow diagram summarising the procedure.
As with all EMA interactions, this procedure follows a published timeline, however there are multiple opportunities for Applicants to have additional informal meetings with the EMA scientific officer or members of the qualification team, to allow a greater degree of collaboration and discussion to support the development of the novel methodology. Furthermore, clock stops can be introduced if the Applicant requires more time to provide additional information. In this regard, the novel methodologies procedure offers more flexibility than other advice procedures, however the potential variability in timelines should be taken into consideration when planning for this request.
Figure 1: Procedure for the Novel Methodologies Qualification Procedure
(taken from EMA/CHMP/SAWP/72894/2008)
FEES & FEE REDUCTIONS
As of 19th June 2020, the fee associated with the novel methodologies procedure is EUR 89,000. A fee reduction of 90% is available for Applicants defined as micro, small or medium-sized enterprises (SMEs), under the EC Recommendation (2003/361/EC) and EC Regulation (EC 2049/2005). Enterprises may be designated an SME if established within the European Community, and if they meet certain thresholds for headcount and financial criteria. Enterprises which are not EU-based but meet the other criteria may be designated as SMEs by appending to an EU-based regulatory consultancy, thereby allowing them to access the fee incentives without needing to set up an EU entity.
OVERVIEW OF CHMP QUALIFICATION OPINION
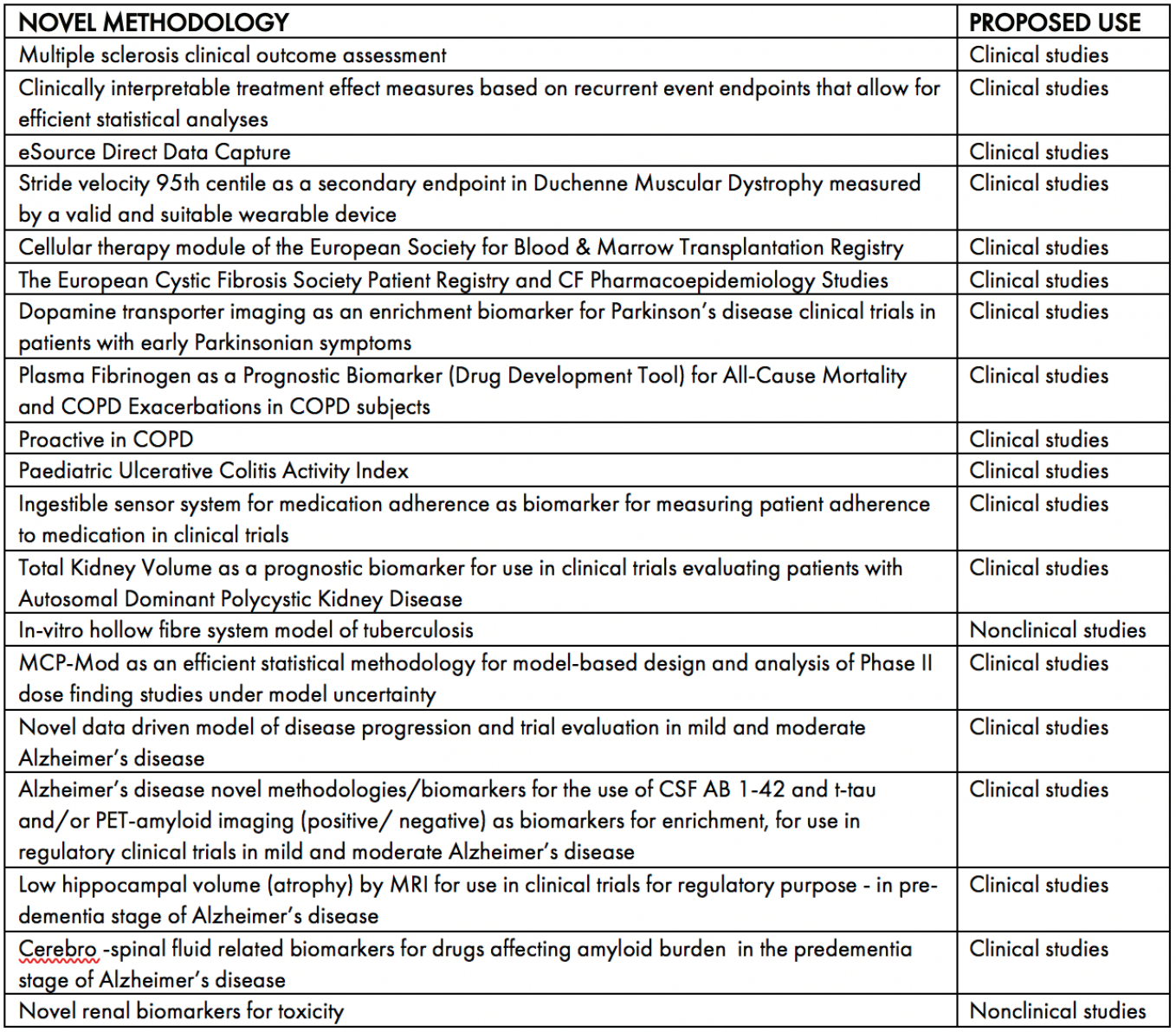
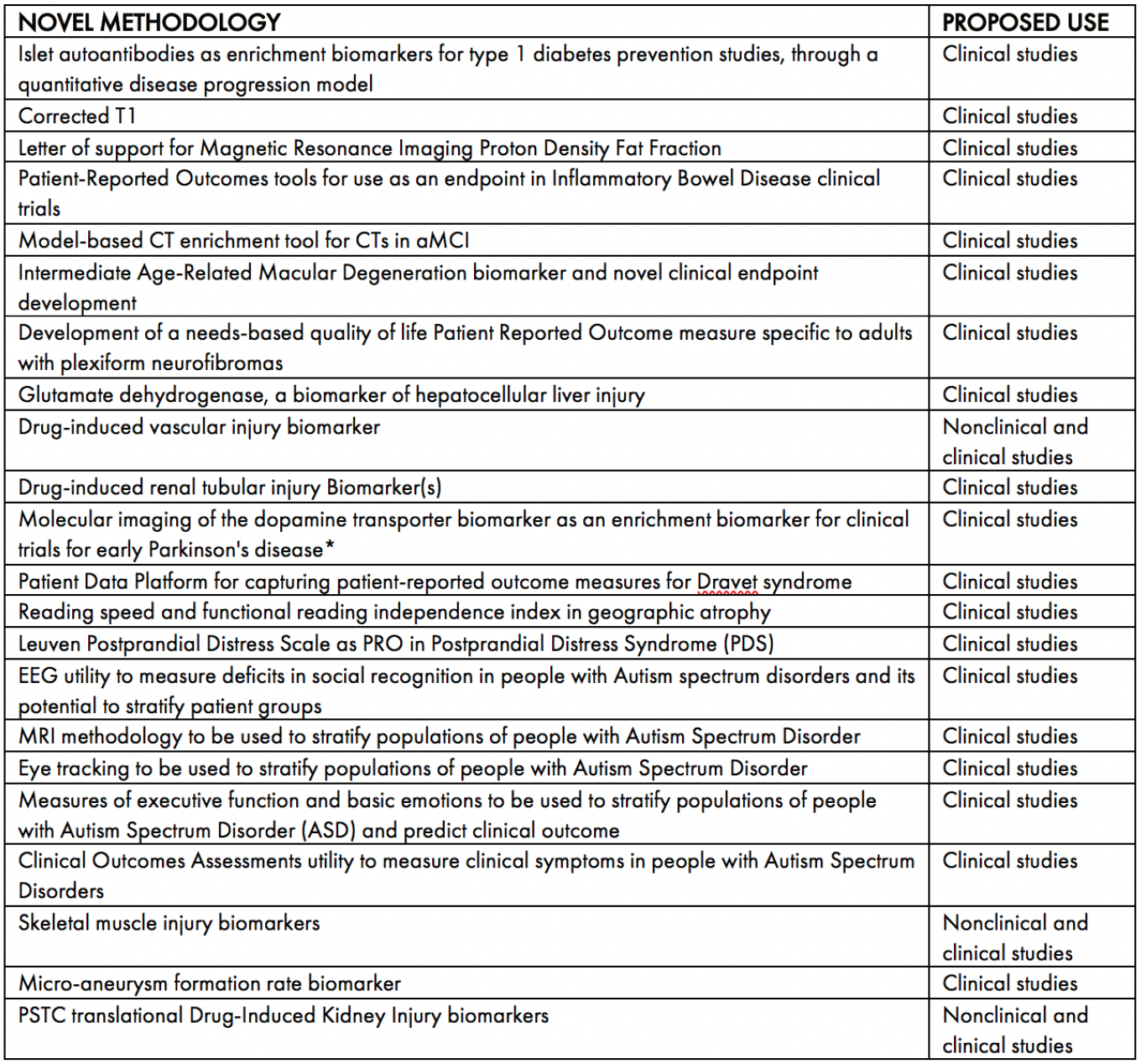
All qualification opinions are made publicly available. As of July 2020, 19 qualification opinions have been given by CHMP. Please see Table 1 for an overview of these opinions. As can be seen, the overwhelming number of the opinions relate to clinical development and indicates the advantage of seeking a qualification opinion to accelerate the clinical phase of medicine development.
Table 1: CHMP qualification opinion
OVERVIEW OF CHMP LETTERS OF SUPPORT
As of July 2020, the CHMP has published 22 letters of support to developers of novel methodologies. As with the qualification opinion, the majority of letters of support relate to novel methodologies for clinical use. Of note, one novel methodology that received a letter of support has since been given a qualification opinion by CHMP. Please refer to Table 2 for an overview of the letters of support.
Table 2: CHMP Letters of Support
BENEFITS OF NOVEL METHODOLOGIES QUALIFICATION
As well as the potential to accelerate the overall clinical development timeline for any given product, receiving a qualification opinion from CHMP may be valuable for securing investment and may eventually result in wider use of the novel methodology by industry. Qualification advice is useful for developers to understand the data requirements to support a future qualification opinion, ensuring that appropriate studies are conducted. Furthermore, if a letter of support is published then this may be valuable for fundraising for further development of the novel methodology.
CONCLUSION & SUMMARY
The novel methodologies procedure is well-established and there are numerous resources available for Applicants considering this procedure, including guidance documents published by EMA and existing qualification opinions which are made publicly available. This is not a short procedure to undertake, with duration of at least 160 days for qualification advice and 250 days for a qualification opinion, without taking into account potential clock stops. There are also substantial fees associated with the request unless the Applicant is eligible for the fee reduction available for SMEs. However, the qualification procedure can be a valuable interaction for developers of novel methodologies for use in drug development, whether the outcome is advice or an opinion, and it is worthwhile for developers to consider communicating with the EMA early in development.