BIA - MHRA Regulatory Innovation Conference 2020.
by Harriet Thomasson
INTRODUCTION
Scendea were delighted to attend the BIA-MHRA Regulatory Innovation Conference 2020 on 17th September. This conference, attended by The CEOs of MHRA, BIA, and the Minister for Innovation, Lord Bethell, focused on the future of the UK regulatory landscape in the context of Brexit and lessons learned from the COVID-19 pandemic.
Returning themes throughout the day included:
Ensuring the UK provides an attractive destination for clinical trials;
Developing a more interactive MHRA with expedited processes for Sponsors;
Including patients in the trial design process, and incorporating the use of real-world data to reduce the burden on trial participants.
Throughout the course of the conference, MHRA announced a number of initiatives to support sponsors, including a new approval pathway for innovative products, a new streamlined licensing procedure for biosimilars, a new informal preassessment service for novel clinical trial designs, and upcoming collaboration with regulatory authorities worldwide.
FUTURE VISION
INTERACTIVE AND RESPONSIVE REGULATION FOR EXPEDITING PATIENT ACCESS.
Keynote address: Future Ambition for UK Life Sciences in 2021 and Beyond
In the keynote address, Lord Bethell, Minister for Innovation, encouraged the use of learnings from the COVID-19 pandemic to inform the future of the UK regulatory landscape post-Brexit, and praised MHRA’s ongoing pragmatic approach to approval of novel trial designs in the wake of COVID-19. He remarked that it would be a mistake to step back into the rigid framework in use prior to the pandemic and noted that the UK’s upcoming secession from the EU provides a unique opportunity in this regard.
The upcoming new medicines and medical devices bill aims to increase flexibility moving forwards, aiming to serve as a foundation for the UK as a first-tier destination for clinical trials, research, and marketing post-Brexit.
The UK Innovation Ecosystem - A Transformed Regulatory Role
Dr June Raine CBE, CEO of MHRA, clearly outlined her mandate for transformation within the agency, aiming to maintain the fast moving, risk-proportionate, independent scientific decisions that are being demonstrated throughout the ongoing response to COVID-19. The key shifts in MHRA’s approach are outlined as follows:
From “Controller” to “Enabler” with no loss of high standards of safety and independence, while still retaining powers for control when needed;
Acting as a global voice, leading in development of innovative regulatory approaches to new technologies, and making the UK the best possible environment for clinical trials;
From “Population Health” to “Patient Champion”, using regulation to influence clinical practice and help patient decision-making, and moving to a “Life Cycle” regulatory approach using real world data and becoming more responsive to patient safety;
From “Black Box” to “System Partner” using joint working with NICE, and conferring real pull-through to the NHS, supported by the care quality commission (CQC).
These four key shifts in MHRA’s approach will be supported by transformation in the assessment of innovative products, such as biologicals, advanced therapies, and gene therapies, as well as vaccines, in vitro diagnostics, and AI algorithms and analytics. New regulatory processes will maximise the use of real-world experience and optimise trial design, with patient engagement increased throughout the decision-making process.
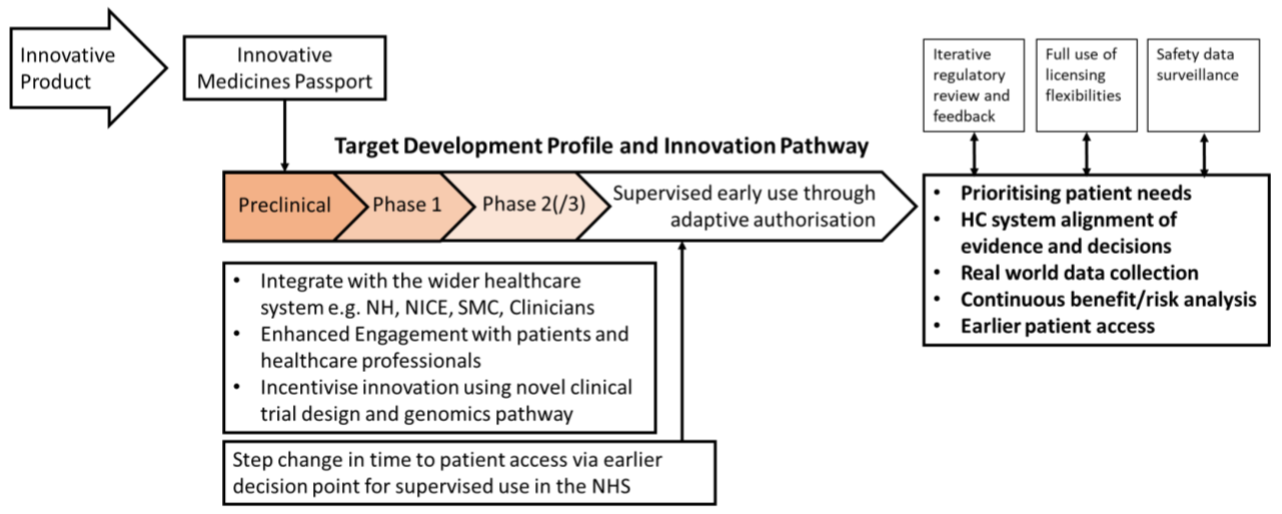
The proposed changes aim to confer efficient and timely medicines development building upon the headline item: a new licensing pathway available from 1st January 2021. This “Target Development Profile and Innovative Pathway” or “Innovative Medicines Passport” (nomenclature to be decided) aims to provide a flexible, integrated pathway that draws on the expertise of MHRA in collaboration with partners from across the wider healthcare system, including NICE. An overview of the new route to authorisation is presented in Figure 1.
Figure 1: New Licensing Pathway for Innovative Products
When questioned, MHRA were unable to clarify the exact eligibility criteria for this pathway but noted that they are examining existing CHMP guidance and aiming to be as inclusive as possible. Meindert Boysen, Deputy Chief Executive of NICE added that the organisation is hoping to take a collaborative approach with Sponsors in defining what constitutes an “innovative medicine”. It was also clarified that this new system would not replace the existing early access to medicines scheme (EAMS), but rather build on it.
MHRA also touched upon their proposed innovative new UK licensing procedure for biosimilar products. The main aim of this change will be to reduce burden on clinical trial data generation following Brexit. In most cases, a comparative efficacy trial will not be considered necessary, given appropriate justification. Sponsors will be encouraged to discuss this approach with MHRA when sufficient comparative analytical and functional data are available. Although minimal details are available at time of writing, the relevant MHRA guideline will be released for public consultation in the coming weeks.
International partnerships were raised as a key area for development following the UK’s withdrawal from the EU. The existing partnerships between MHRA and global regulators, including those in Australia, Canada, Switzerland, and Singapore, will continue to be strengthened, while also forging new ones. Based on comments made by Dr Raine when questioned on this topic, MHRA are expected to announce closer collaboration with US FDA in the near future.
Facilitating Access to Innovation - NICE’s New Partnership with MHRA
Meindert Boysen, Deputy Chief Executive of NICE, announced a new formal partnership between NICE and MHRA, developed from their close working during the COVID-19 pandemic. The first output of this collaboration (developed with input from the Scottish Medicines Consortium, SMC) is the new innovative licensing pathway pilot discussed by Dr Raine in the previous presentation. It was noted that this new approach to licensing and access does come with challenges, including:
Downstream complexity created by early licensing, such as having less mature evidence on which to base a clinical and cost effectiveness evaluation, impacting the HTA and commissioning processes;
Ensuring safe and financially sustainable early patient access, which will require policy solutions, such as managed access arrangements (where financial risks to the NHS are managed in parallel with further evidence development);
Closing evidence gaps following the early launch of promising medicines will require the commissioning and interpretation of real-world evidence studies.
NICE discussed possible solutions to the points above, including multi-stakeholder participation to manage possible downstream effects of earlier licensing, and application of NICE’s experience as part of major international real-world evidence research collaborations and their in-house data and analytics capability. It is expected that the cancer drugs fund will soon be broadened into the innovative medicines fund, allowing these approaches to be applied beyond the current oncological scope.
Patient Expectations of the Innovation Agenda - How Can These be Met?
Aisling Burnand, MBE, Chief Executive of the Association of Medical Research Charities, discussed the future of clinical trial design, aiming to shift the focus onto reducing patient burden. The key principal of this approach is for Sponsors to engage patients throughout the trial design process. It was noted that patient input can be invaluable in trial design, for example ensuring trial visits are in line with regular check-ups, and thus reducing patient burden.
It was also raised that future research should be more representative of the UK population, with re-examination of common inclusion and exclusion criteria to prevent trial enrolment from being overly restrictive. Under the current paradigm, it was opined that the lack of diversity and equality of access to clinical trials means that many datasets are essentially incomplete and unrepresentative. It was noted that patients are also expecting better feedback from trials in which they have participated, and updates on the overall findings from said trials.
To deliver these changes, it was proposed that regulators build stronger relationships with patients and the public, with FDA pioneering a model of agency forums with affected patients. Greater collaboration across the sector would also be of benefit, with the Association of Medical Research Charities keen to facilitate patient interactions. Finally, the expertise of patients was recognised, with researchers encouraged to take advantage of their deep understanding of the disease and associated patient pathway.
INTEGRATING INNOVATIVE APPROACHES TO CLINICAL DEVELOPMENT IN THE UK
Innovative Clinical Trial Platform - Moving on from the COVID-19 Experience
Dr Kirsty Wudenbach, Deputy Manager of the Clinical trials unit at MHRA, spoke to her personal involvement with the response to COVID-19, during which an informal rolling review process for Clinical Trial Applications (CTAs) was introduced, and advice meetings were provided at short notice. During this time, faster trial approval was achieved, with no compromise in the robustness of review. The emergence of COVID-19 also highlighted the need for greater flexibility around novel trial designs, an area that MHRA was already heavily involved with before the pandemic.
To build upon this momentum, MHRA will now offer a pre-assessment service for Sponsors of designated clinical trials to assist with finalisation of the key documentation (protocol, IB, IMPD) required for submission of a CTA. As part of this new service, the Clinical Trials Unit assessment team will provide feedback in an expedited timeframe to facilitate document finalisation and internal sign offs prior to the project entering the critical path. The aim of this service will be to greatly improve the chance of an application receiving a CTA without additional requests for further information at the time of the formal submission.
Additionally, MHRA is keen to provide regulatory assurance on innovative design elements, such as virtual trial conduct. It was acknowledged that while scientific support is already in place, there is room to stretch these ideas further, as follows:
Early engagement support and advice jointly with HRA and others, where appropriate (via MHRA Innovation Office);
Promotion of early engagement throughout the development lifecycle, as part of a wider more flexible regulatory system;
Continued streamlining of reviews/approvals with HRA, such as the existing Combined Ways of Working scheme;
Greater consideration for how the patient voice can be heard.
This integrated approach to support innovation in study design will be fundamental in the development of MHRA’s proposed “regulatory toolkit” which aims to offer Sponsors individual procedures that can be selected to support a bespoke development programme.
During the panel discussion at the end of this session, it was queried if MHRA had considered a US FDA IND-style approach to clinical trial approvals, to reduce the regulatory burden on industry. MHRA were clear that due to the strong non-commercial aspect of the UK biosciences sector (to whom an IND approach is less suitable), an IND-style approach, if considered, would not be rolled out universally.
How Can We Build on NIBSC’s Unique Role in UK Stem Cell Research and ATMP Development?
Dr Christian Schneider, Director of the National Institute for Biological Standards and Control (NIBSC), discussed how the state-of-the-art facilities available at the UK Stem Cell Bank (UKSCB) could be better used in future as the number of trials involving stem cells continues to rise. It was noted that the UK banks almost half the world’s stem cell lines, representing a unique resource and opportunity.
One clear benefit of the use of UK Stem Cell Bank cGMP cell lines is the availability of a CMC information package, comprising of the routine QC testing carried out. This data package is suitable for CTAs and is a real-world example of the application of regulatory science. Key among the testing work carried out is UKSCB’s research to understand batch-to-batch variability and assess phenotypic drift throughout the manufacturing process.
The UKSCB was recognised as a model for regulatory science, and an integral part of the UK’s strong landscape and infrastructure for Advanced Therapies. It was advocated that the MHRA should continue evolving at a fast pace to provide an attractive environment for the development of ATMPs.
Dr Trevor Walker, Head of Regulatory Affairs at IQVIA, identified three key aspects that together provide an optimal environment for a Sponsor to conduct a clinical trial:
A supportive regulatory environment, including a knowledgeable and easily accessible regulatory authority that has expertise with innovative trial designs;
A streamlined and efficient process for the authorisation of clinical trials;
The availability of experienced clinical research sites with a proven track record, that can efficiently identify and recruit subjects and produce high quality clinical data.
MHRA was praised for its streamlined approach to clinical trial authorisation, alongside its expertise, accessibility, and strong reputation. MHRA’s relationships with FDA and ICH are considered critical by industry to maintain this reputation after withdrawal from the EU.
The support for remote trials provided by MHRA during the response to COVID-19 has been widely welcomed by the industry, along with their commitment to early engagement. The broad range of interactions available throughout the product development life cycle is considered a distinct advantage, and it was noted that IQVIA often uses MHRA opinion as a gauge for broader regulatory acceptance. In particular, the industry is keen to see a continuation of the supportive regulatory environment and expedited review procedures demonstrated to be possible during the COVID-19 pandemic.
The utility of the Combined Ways of Working (CWOW) initiative where submission of one set of documents to MHRA and HRA with harmonised review has the effect of accelerating CTA review timelines and ultimately conferring faster trial initiation was noted and advocated. This scheme has been running for approximately 2 years as a pilot for the EU Clinical Trial Regulation (CTR). Following the UK’s withdrawal from the EU, the expectation is that CWOW and the EU CTR will have close alignment.
Based on the previous points, and in particular the availability of accelerated timelines, it was considered likely that the UK will remain a competitive trial destination. In terms of patient recruitment and site activation, the UK is one of the most efficient countries in Western Europe.
It was raised that the UK should aim to compete with Eastern European countries in both regards, through improved patient recruitment and expediting site activation.
To drive the UK forwards as an attractive clinical trial destination, the following approaches were considered key:
Improved ability to find and deliver the study population, for example through the use of electronic medical records to directly contact patients who may benefit from a particular research study;
Increased patient engagement, through ensuring trials are patient-centric (e.g. using virtual trial strategies) and timely sharing of research results to demonstrate the value of their contribution to future healthcare;
Normalisation of research across all care settings, by integration of clinical research across NHS activities, with dedicated resources and further promotion of existing UK research networks, alongside a standardised process for financial and contractual engagement.
Overall, the existing UK regulatory landscape provides an attractive environment for the conduct of a clinical trial, through the flexibility, efficiency, expediency, and collaborative approach demonstrated by MHRA. However, it was remarked that future transformation of the clinical research environment as discussed will benefit industry and further promote the UK as a destination for clinical research. During the panel discussion, access to electronic medical records was identified as a potential gamechanger in the recruitment of patients to studies which could be of benefit to them.
REAL WORLD DATA:
WHAT IS POSSIBLE AND WHEN?
A New Vision for the Clinical Practice Research Datalink Services in Innovation
Dr Janet Valentine, Director of the Clinical Practice Research Datalink (CPRD), introduced CPRD as the UK Government health data research service supporting observational & interventional public health and clinical studies by academics, industry and regulators worldwide. The service collects data daily from the NHS and covers 24% of the UK population. Notably, the median availability of patient follow-up data is 10 years, with 20 years follow-up data available from 25% of patients. These primary care records provide treatment, laboratory, event, and adherence data, and have been used in support of numerous observational studies.
The electronic health records (EHRs) stored within CPRD can be used in a number of manners to improve the progress of a clinical trial, for example in informing protocol design and feasibility, selecting and locating eligible patients, and targeting site selection.
In selecting eligible patients, CPRD screen their database against the inclusion and exclusion criteria of a given trial, and progress as follows:
Central search of CPRD primary care database for eligible patients;
Contact CPRD network of 1900 GP practices;
GP reviews eligible patients for suitability;
GP invites only suitable patients;
High quality patients contact study centre.
Throughout the process, only the GP has access to identifying patient details, ensuring confidentiality is maintained. A recent example demonstrated that the EHR search can be modified in real time to reduce the rate of screening failures. Once a trial has been initiated, CPRD is able to randomise patients within the system and monitor them throughout.
The application of CPRD to digital technologies has been explored through the development of a synthetic data set, jointly by MHRA and CPRD. The aim of this project was to develop a synthetic dataset for validation of clinical decision-making algorithms to ensure AI software is safe and appropriately tested. Creation of high-fidelity synthetic data was demonstrated, based on fidelity synthetic data based on cross-sectional anonymised primary care data, capturing complex clinical relationships while being 100% artificial.
The potential applications of synthetic data to support clinical trials include:
Scale up of data when larger sample sizes are needed;
Conditional generation of data fields to address imbalances and minimise bias;
Generation of synthetic patient cohorts to support in silico trials to simulate intervention effects in sub-groups not typically included in RCTs (for example, pregnant women);
Provision of external control groups for clinical testing or benchmarking data from a single-arm trial.
In the future, CPRD aim to use this technology to provide a customised synthetic data generation service, available to industry.
During the panel discussion, it was noted that synthetic data is at a very early stage, and CPRD are the first to validate synthetic data sets for missing data. Metadata and transparency are the core elements that regulators are looking for in both synthetic and real-world data. MHRA will be releasing guidance on the use of the real-world data in the near future.
How Can We Maximise the Use of Real-World Data in Delivering Innovation?
Dr Alison Cave, Challenge Director of the Industrial Strategy Challenge Fund (ICSF), identified a number of regulatory and healthcare challenges currently faced, including an increasing understanding that a traditional drug development pathway does not fit all medicines, leading to uncertainties in post-authorisation regulation.
Real World Data (RWD) was presented as a possible solution to this and other problems, with the caveat of multiple uncertainties. For example, RWD is produced for clinical care delivery rather than research, and as such records are subject to systematic and random error.
These issues were summarised in the following problem statement:
“Advances in information technology are driving digitisation of large volumes of research and clinical data, commonly termed big data. While the capture and analysis of these data offer possibilities to better characterise disease, treatments and the performance of medicinal products in individual healthcare systems, the acceptability of such insights as evidence for decision making is uncertain.
The data in itself does not provide value. It needs to be analysed and interpreted and ultimately acted upon for the value to be realised. Methodologies and analytical approaches which reveal patterns, trends and associations are equal partners in generating evidence from big data but causality cannot be assumed.”
As such, the challenge set out is to capitalise on the promise of novel datasets of unknown quality while still reaching a robust position on the benefit of a medicine. It was proposed that the recommendations of the HMA-EMA big data taskforce would provide a suitable solution, particularly with regards to data acceptability and data governance.
The discoverability of relevant data was set out as a clear prerequisite, and the new Health Data Research (HDR) Innovation gateway highlighted, currently providing a consolidated searchable database of 483 datasets. A benefit of the HDR is the visibility of data relevance, achieved by clear fingerprinting of datasets within HDR.
It is clear that the capture and analysis of RWD offer possibilities to better characterise disease, treatments and the performance of medicinal products in individual healthcare systems, but the acceptability of such insights as evidence for decision making is uncertain. Multiple challenges still exist in understanding the reproducibility and validity of the derived evidence, so it is critical to have a clear understanding of if the available dataset is fit for purpose.
A Product Life-Cycle Approach to the Use of Real-World Data
Simon Bennet, Director of Regulatory Policy at Biogen noted that RWD has a role throughout the product life cycle, but most notably during the post-licensing period. The use of RWD still faces challenges, and as such regulators should aim to provide greater insight into how RWD data is viewed, following the lead of the US and Chinese FDA. The need for a consistent approach from regulators across data sources was highlighted.
Possible solutions to the challenges of ensuring data relevance, quality and patient privacy were discussed and included:
Building acceptance of new sources of data for high quality evidence generation that supports regulatory and HTA review, and holistic review of value of medicines;
Improvement of the quality, scientific relevance and interoperability of source RWD;
Development of best practices in study design and analytical approaches used in generating evidence from RWD;
Achievement of sustainable, appropriate industry access to RWD that respects data privacy concerns.
With these issues addressed, RWD can be integrated into an evidence generation plan, drawing on research questions, data gathering, and methods and analytics to provide insights and evidence.
In future it is hoped that common data models and enhanced collaboration and coordination will increase value and use of RWD, enabling contribution to the development of medicinal products across their lifecycle.
CLOSING REMARKS
To close the conference, both Dr June Raine CBE, CEO of MHRA, and Steve Bates, CEO of BIA, gave their thanks to the speakers and attendees. The key themes of the day were summarised, with the need for transformation of innovative medicines regulation restated. It was stated that a reset for clinical trials, cell-based therapies, and data access is required, with a focus on including patients in trial design from the outset.
To this end, MHRA are implementing the new innovation passport licensing pathway, early advice interactions and international collaboration. News on all of these items will be available soon.